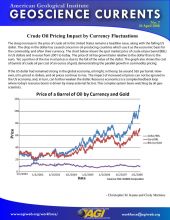
Mineral Commodity Spotlight
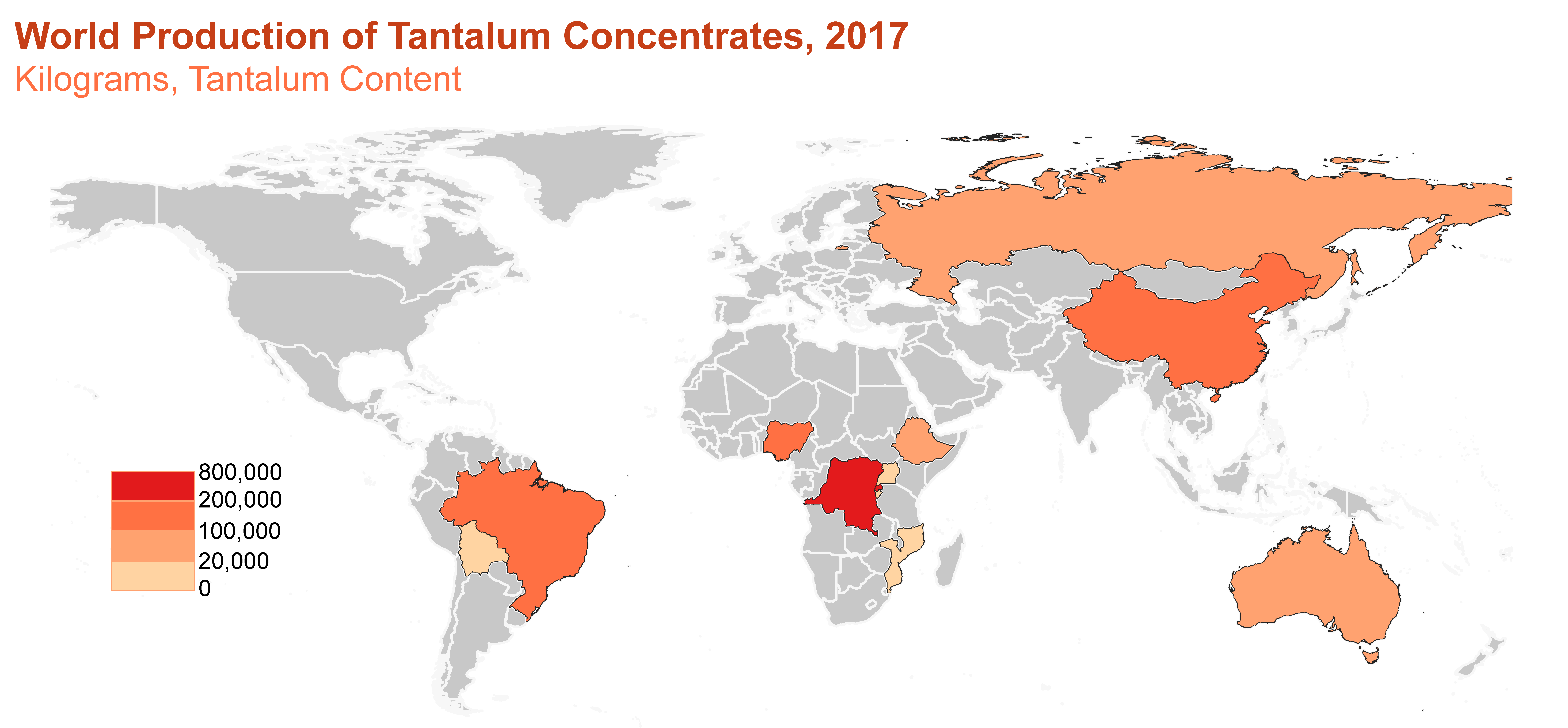
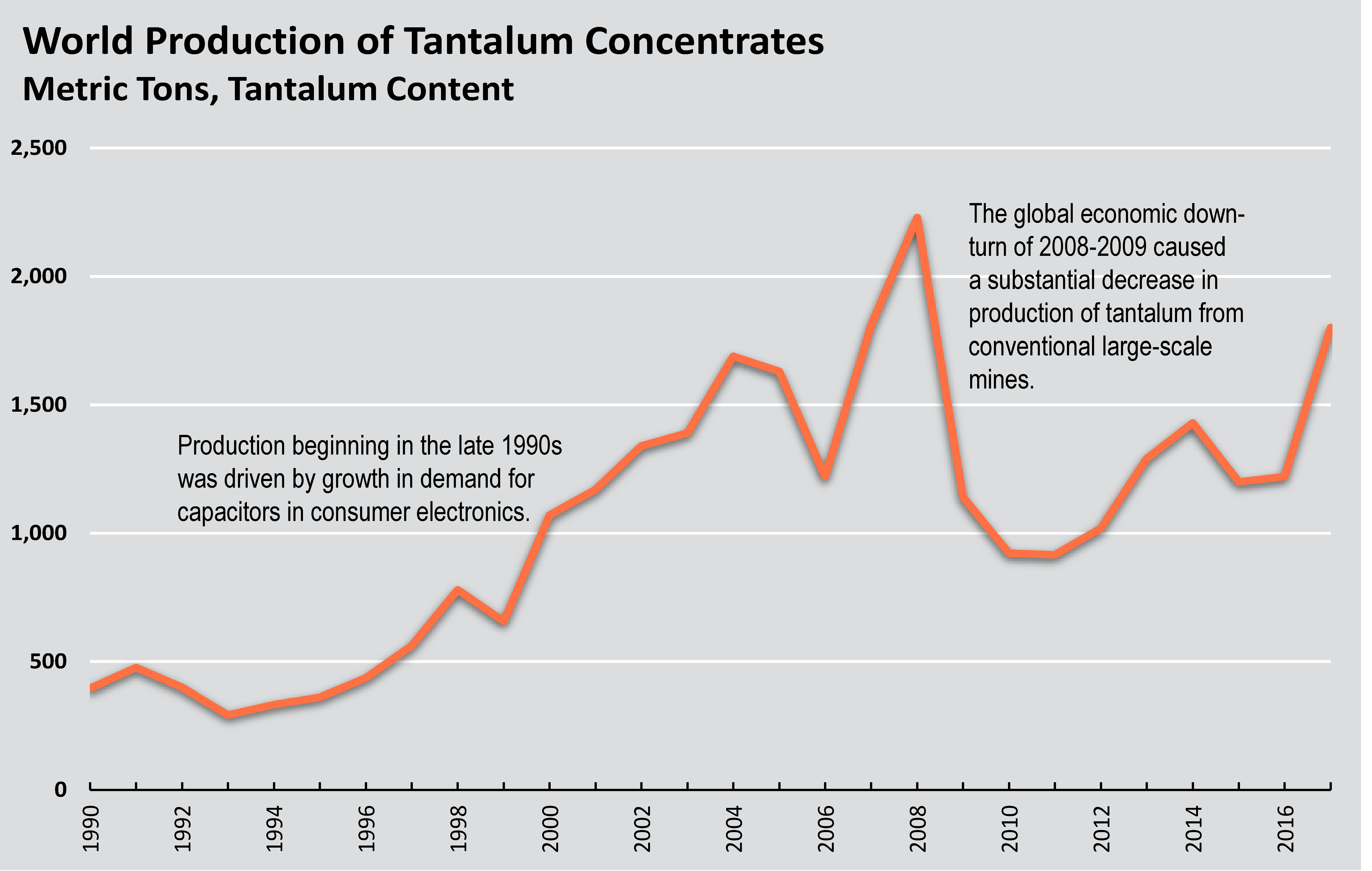
Production
Geologic Occurrence
Tantalum, a rare, very hard transition metal, does not occur naturally in metallic form. The estimated average abundance of tantalum in Earth's crust is about 2 parts per million (or 0.0002%). Although many tantalum-bearing minerals have been identified, the most economically significant ones are the oxide minerals columbite, microlite, tantalite, and wodginite. Most of the world's largest tantalum resources are associated with rare-metal pegmatites, such as those found in the Pilbara Craton (Australia) and pegmatites of the Kibaran orogen (Africa). Tantalum also occurs in some rare-metal granites, and in placer deposits derived from weathering of those granites and pegmatites.
World production of tantalum concentrates. Source: U.S. Geological Survey.
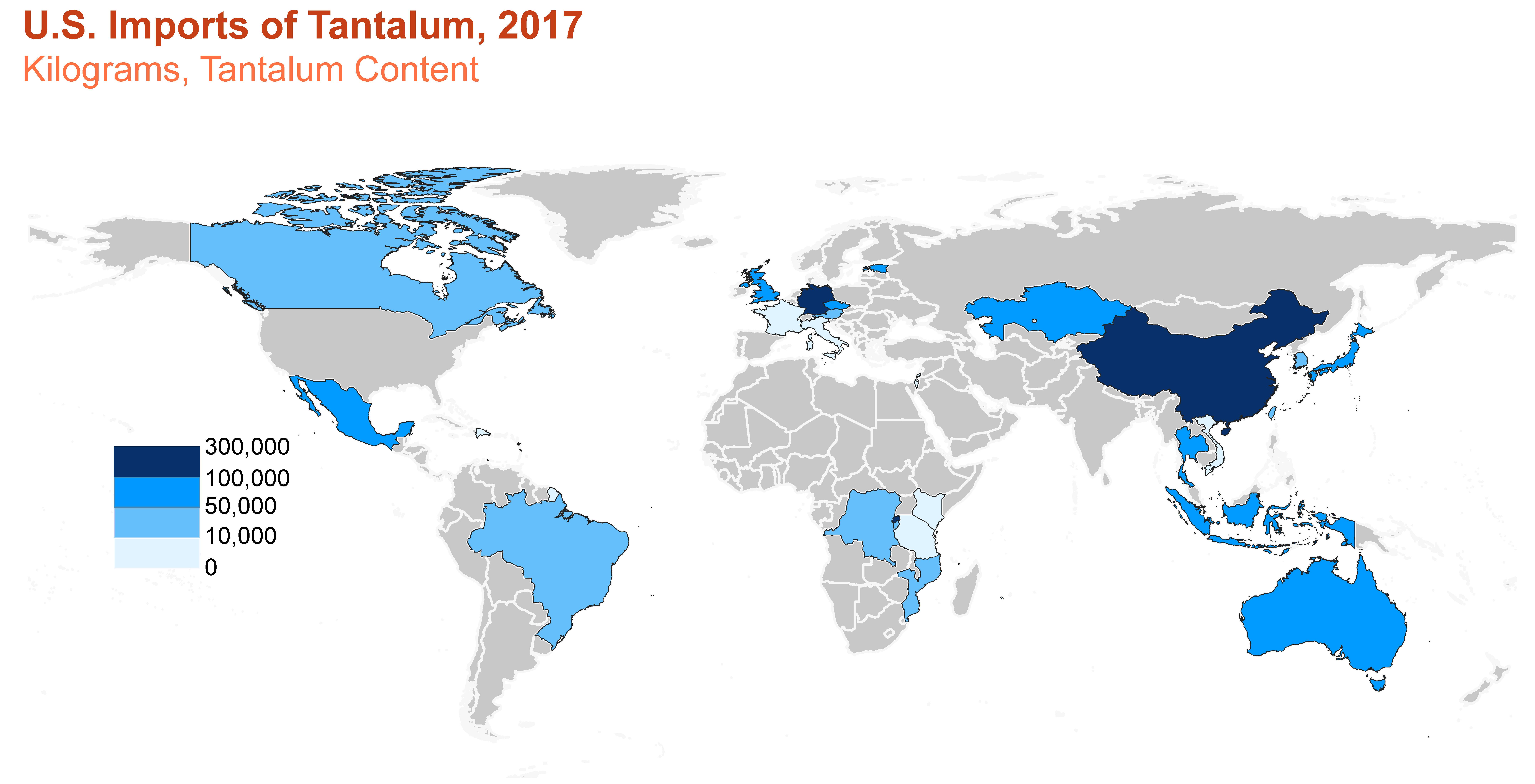
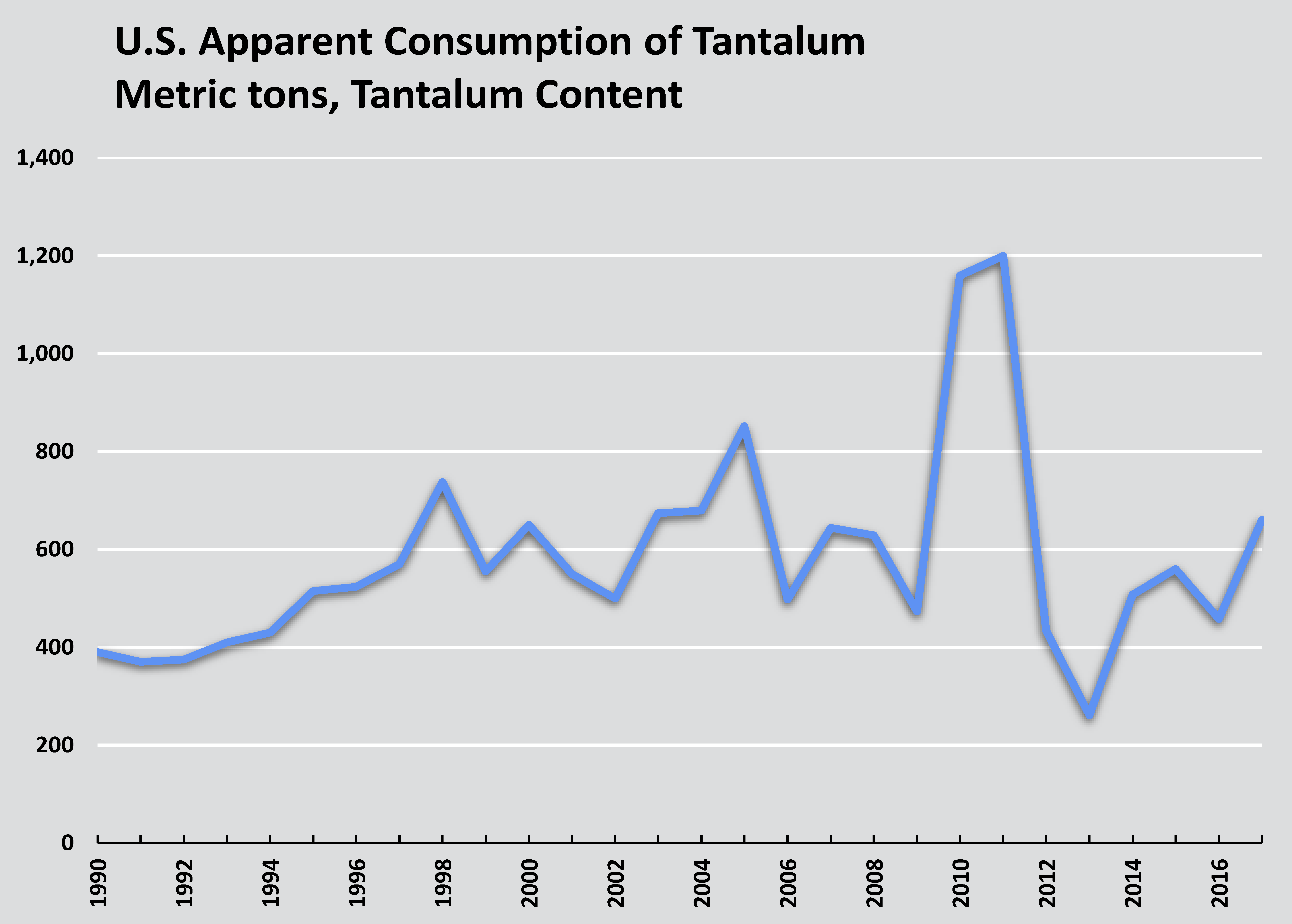
Consumption
Commercial Usage
Tantalum is largely used in applications that require increased chemical, corrosion, and/or heat resistance. Roughly half of the tantalum is consumed by the electronics industry, primarily to manufacture capacitors (for electronic products such as cellular phones and computers) and sputtering targets. The remainder is consumed by the chemical and metallurgical industries to manufacture tantalum-containing carbide cutting tools, chemicals, and superalloys.
U.S. imports and apparent consumption of tantalum. Source: U.S. Geological Survey.
Fun Facts
Of the elements with stable isotopes, tantalum is estimated as the least abundant in the entire universe. Only 3 other metals have higher melting points than tantalum metal (3,017ºC): osmium (3,033ºC), rhenium (3,186ºC), and tungsten (3,422ºC). Tantalum is inert and biocompatible (does not react with body fluids) and therefore used for making surgical equipment and medical implants.
This Commodity Spotlight can be freely distributed in its entirety. Any modification requests should be submitted to ckeane@intraw.eu.